Acute inflammation indicator - CRP
Acute inflammation indicator - CRP:C-reactive protein (CRP) is a classic acute-phase reactive protein first identified in the blood of patients with Streptococcus pneumoniae infection. It is named for its ability to bind to the phosphocholine residue (Pch) of the C-polysaccharide in the capsule of Streptococcus pneumoniae in the presence of calcium ions (Ca²⁺). Under normal conditions, CRP levels are extremely low, but after stimulation by acute inflammatory responses, serum CRP can significantly increase to 100–1000 times normal levels.
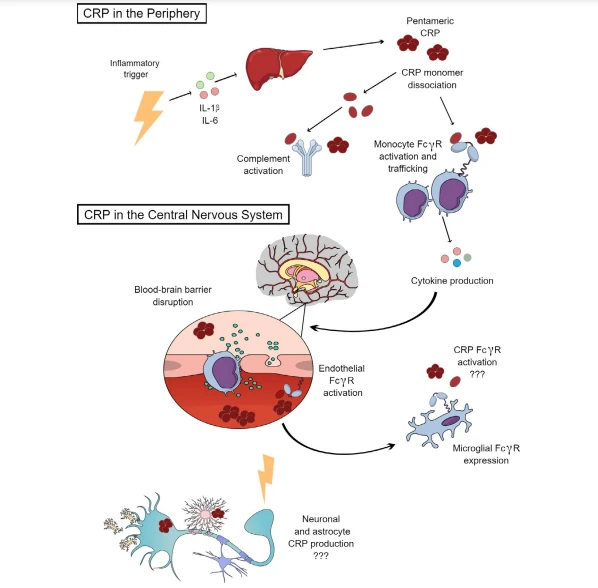
Physiological Functions of CRP:In the presence of Ca²⁺, CRP can bind not only to pathogens and Pch on the membranes of apoptotic/necrotic cells but also to nuclear components such as histones, chromatin, and small nuclear ribonucleoproteins. CRP is primarily produced in the liver, with minor contributions from neurons, atherosclerotic plaques, lymphocytes, and adipocytes (which have little impact on serum levels). In the liver, its synthesis is transcriptionally regulated by the cytokine interleukin-6 (IL-6), with IL-1β synergizing with IL-6 to enhance CRP production.
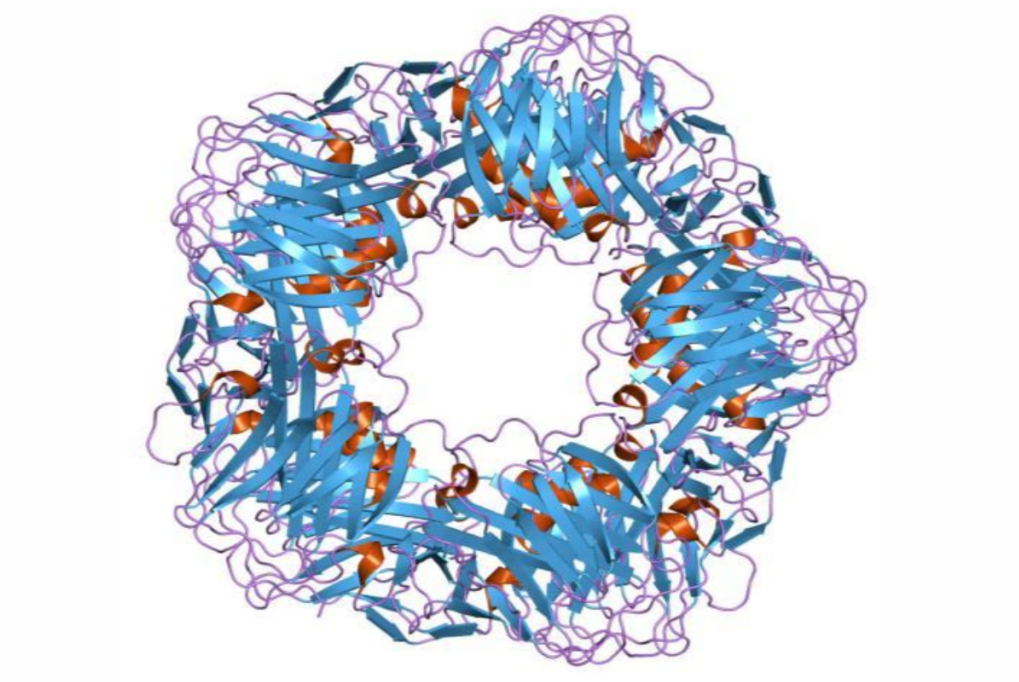
Produced CRP can bind to complement C1q, activating the classical complement pathway to regulate pathogen phagocytosis. It also interacts with the Fc segment receptors (FcγRⅠ and FcγRⅡ) of human immunoglobulin G, triggering phagocyte responses that lead to bacterial or cellular lysis. Closely linked to inflammation, CRP can act as both a pro-inflammatory and anti-inflammatory molecule through structural transformations. Pentameric CRP has pro-inflammatory functions alongside weak anti-inflammatory bioactivity, while monomeric CRP exhibits strong pro-inflammatory bioactivity.
CRP production begins 4–6 hours after inflammatory stimulation, doubling every 8 hours and peaking at 48 hours.
Applications of CRP in Infectious Diseases:Infectious diseases, caused by pathogen infections, are broadly categorized into bacterial and viral infections. A CRP level >100 mg/L is considered an indicator of infection and aids in differential diagnosis: bacterial infections rapidly induce significant CRP elevation, whereas viral infections cause only minor increases.
Applications of CRP in Autoimmune Diseases:CRP levels rise during the active phase of most autoimmune diseases, with prominent applications in rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). RA is a systemic chronic inflammatory disease leading to joint destruction. As a marker for RA, CRP promotes bone destruction in RA by inducing osteoclast differentiation factor expression and direct differentiation of osteoclast precursors into mature osteoclasts. Sustained CRP elevation indicates a high risk of progressive joint deterioration, necessitating more aggressive treatment and care; reducing CRP levels is critical in RA management.
Applications of CRP in Oncology: Inflammation promotes cancer initiation, progression, and metastasis, with chronic inflammation playing a key role in malignant tumor pathogenesis. Cancer patients exhibit higher plasma CRP levels than healthy populations, as tumor necrosis factor induces hepatic CRP synthesis. Elevated plasma CRP is a marker of cancer risk in healthy individuals and correlates positively with the incidence of head and neck cancer, esophageal cancer, gastric cancer, colorectal cancer, liver cancer, lung cancer, breast cancer, renal cancer, and non-Hodgkin lymphoma. CRP also serves as a survival predictor for tumor prognosis and is recognized as a marker for cancer cachexia due to its role in regulating muscle cell proliferation and metabolism.
Applications of CRP in Cardiovascular Diseases:High-sensitivity CRP (hs-CRP) is a reliable and independent risk marker for cardiovascular diseases, with elevated levels indicating increased risk. Cardiovascular risk is stratified into low, moderate, and high categories based on hs-CRP levels (<1 mg/L, 1–3 mg/L, >3 mg/L).
Conclusion :As a nonspecific marker of inflammatory responses, CRP levels change in infections, autoimmune diseases, surgical trauma, cancer, and cardiovascular diseases, making it a valuable tool for disease assessment.
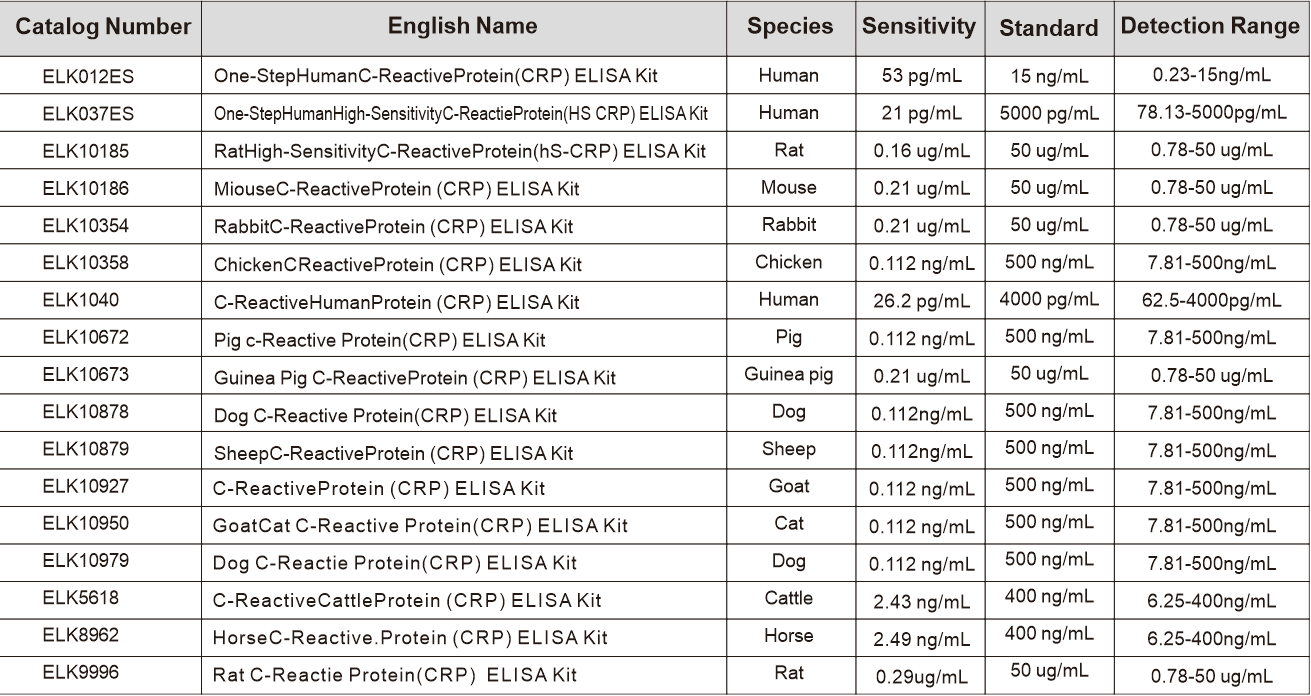
Related ELK Products