| Product name: |
KDEL Receptor 3 rabbit pAb |
| Reactivity: |
Human;Mouse |
| Alternative Names: |
KDELR3; ER lumen protein retaining receptor 3; KDEL endoplasmic reticulum protein retention receptor 3; KDEL receptor 3 |
| Source: |
Rabbit |
| Dilutions: |
Western Blot: 1/500 - 1/2000. Immunohistochemistry: 1/100 - 1/300. ELISA: 1/20000. Not yet tested in other applications. |
| Immunogen: |
The antiserum was produced against synthesized peptide derived from human ERD23. AA range:61-110 |
| Storage: |
-20°C/1 year |
| Clonality: |
Polyclonal |
| Isotype: |
IgG |
| Concentration: |
1 mg/ml |
| Observed Band: |
28kD |
| GeneID: |
11015 |
| Human Swiss-Prot No: |
O43731 |
| Cellular localization: |
Endoplasmic reticulum membrane ; Multi-pass membrane protein . Golgi apparatus membrane ; Multi-pass membrane protein . Cytoplasmic vesicle, COPI-coated vesicle membrane ; Multi-pass membrane protein . Localized in the Golgi in the absence of bound proteins with the sequence motif K-D-E-L. Trafficks back to the endoplasmic reticulum together with cargo proteins containing the sequence motif K-D-E-L. . |
| Background: |
KDEL endoplasmic reticulum protein retention receptor 3(KDELR3) Homo sapiens This gene encodes a member of the KDEL endoplasmic reticulum protein retention receptor family. Retention of resident soluble proteins in the lumen of the endoplasmic reticulum (ER) is achieved in both yeast and animal cells by their continual retrieval from the cis-Golgi, or a pre-Golgi compartment. Sorting of these proteins is dependent on a C-terminal tetrapeptide signal, usually lys-asp-glu-leu (KDEL) in animal cells, and his-asp-glu-leu (HDEL) in S. cerevisiae. This process is mediated by a receptor that recognizes, and binds the tetrapeptide-containing protein, and returns it to the ER. In yeast, the sorting receptor encoded by a single gene, ERD2, is a seven-transmembrane protein. Unlike yeast, several human homologs of the ERD2 gene, constituting the KDEL receptor gene family, have been described. KDELR3 was the third member of the family to be identified. Alt |


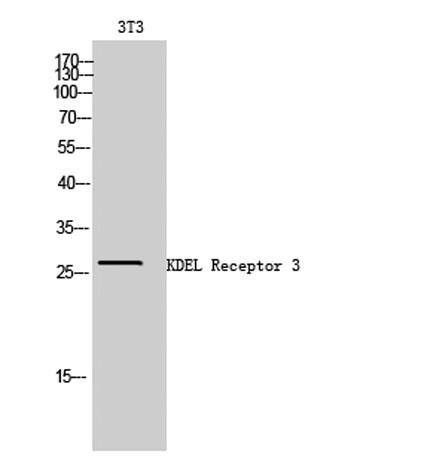



 Western Blot analysis of 3T3 cells using KDEL Receptor 3 Polyclonal Antibody
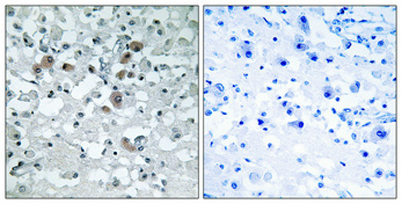
Western Blot analysis of 3T3 cells using KDEL Receptor 3 Polyclonal Antibody Immunohistochemical analysis of paraffin-embedded Human brain. Antibody was diluted at 1:100(4° overnight). High-pressure and temperature Tris-EDTA,pH8.0 was used for antigen retrieval. Negetive contrl (right) obtaned from antibody was pre-absorbed by i
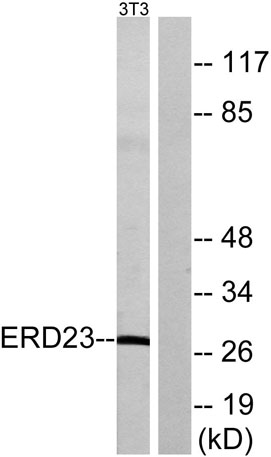
Immunohistochemical analysis of paraffin-embedded Human brain. Antibody was diluted at 1:100(4° overnight). High-pressure and temperature Tris-EDTA,pH8.0 was used for antigen retrieval. Negetive contrl (right) obtaned from antibody was pre-absorbed by i Western blot analysis of lysates from NIH/3T3 cells, using ERD23 Antibody. The lane on the right is blocked with the synthesized peptide.
Western blot analysis of lysates from NIH/3T3 cells, using ERD23 Antibody. The lane on the right is blocked with the synthesized peptide.
 Manual
Manual