



Human HSP-70/HSPA9(HeatShock Protein 70) ELISA Kit
ELK4514| 48 T | $320.00 |
| 96 T | $458.00 |
Overview
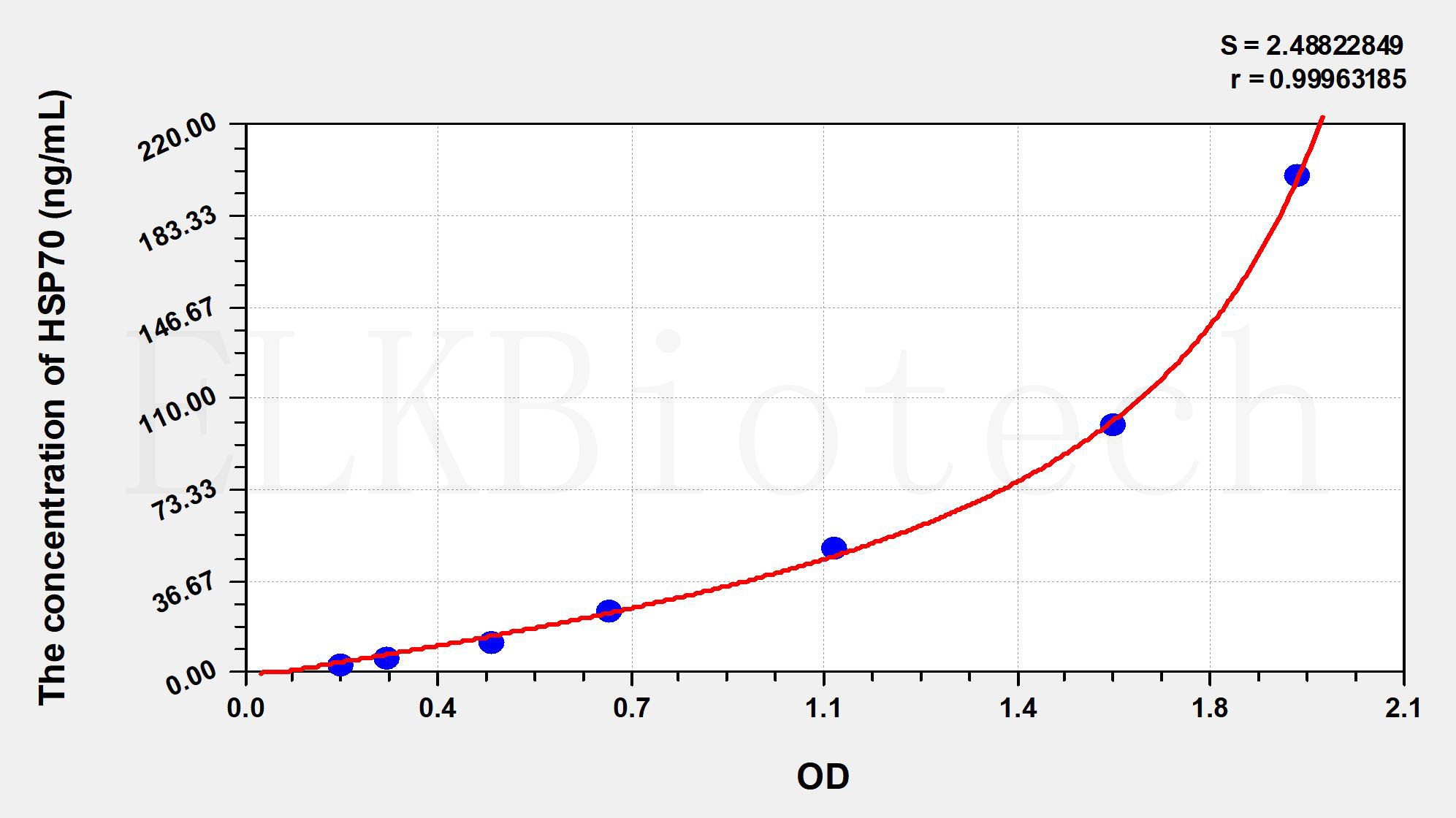
Standard curve
| Concentration (ng/mL) | OD | Corrected OD |
|---|---|---|
| 200.00 | 1.984 | 1.919 |
| 100.00 | 1.647 | 1.582 |
| 50.00 | 1.139 | 1.074 |
| 25.00 | 0.728 | 0.663 |
| 12.50 | 0.517 | 0.452 |
| 6.25 | 0.323 | 0.258 |
| 3.13 | 0.238 | 0.173 |
| 0.00 | 0.065 | 0.000 |
Precision
Intra-assay Precision (Precision within an assay):CV%<8%
Three samples of known concentration were tested twenty times on one plate to assess intra-assay precision.
Inter-assay Precision (Precision between assays):CV%<10%
Three samples of known concentration were tested in forty separate assays to assess inter-assay precision.
Recovery
Matrices listed below were spiked with certain level of recombinant HSP-70/HSPA9 and the recovery rates were calculated by comparing the measured value to the expected amount of HSP-70/HSPA9 in samples.
| Matrix | Recovery range | Average |
|---|---|---|
| serum(n=5) | 82-98% | 90% |
| EDTA plasma(n=5) | 82-94% | 88% |
| Heparin plasma(n=5) | 80-97% | 88% |
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of HSP-70/HSPA9 and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Matrix | 1:2 | 1:4 | 1:8 | 1:16 |
|---|---|---|---|---|
| serum(n=5) | 85-97% | 87-96% | 85-96% | 85-95% |
| EDTA plasma(n=5) | 86-95% | 93-103% | 87-98% | 85-96% |
| Heparin plasma(n=5) | 92-102% | 81-93% | 86-98% | 91-103% |

 Download ①
Download ①